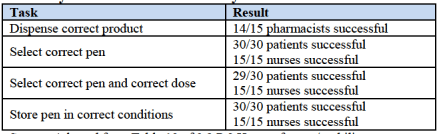
In an attempt to try and overcome this potential hurdle in its NDA, Sanofi submitted data from a human factor trial (HFC) alongside the application. The trial put the pen in front of 60 people (a range of nurses, patients and pharmacologists) and asked them to select the correct dose based on certain individual patient profiles. Here are the results:
At a glance, these don’t look too bad. However, the advisory panel cited concerns surrounding the study relating to how much time participants had to educate themselves as to usage, and suggested that this might not be representative of a real life situation. Further, that a patient out of thirty mis-dosed, and a pharmacist out of fifteen dispensed the wrong pen, is a concern. At the levels Sanofi SA (ADR) (NYSE:SNY) is hoping to sell this product, these numbers suggest thousands of individuals mis-dosing.
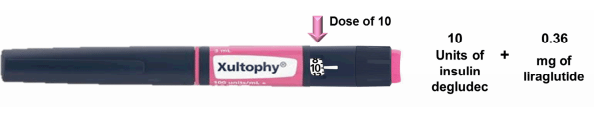
Compare the Sanofi pen to the Novo pen, and the argument for a total redesign strengthens. The image below is the proposed Novo version, taken from the advisory panel review briefing:
One pen for all doses, and an automatic dose of the GLP-1 agonist. Far simpler, and the review panel recognized this fact, with all members voting unanimously in favor of approval. In contrast, the Sanofi review panel came out 12-2 in favor of an approval. Their concerns?
You guessed it:
I WAS CONCERNED WITH THE RESULTS OF THE HUMAN FACTOR STUDY. I DO THINK THE EVIDENCE OTHERWISE SUPPORTS THAT THIS AGENT IS EFFECTIVE AND SAFE IN THE VAST MAJORITY OF PATIENTS. I THINK THE ADVANTAGES OUTWEIGHED THE DISADVANTAGES, OTHER THAN THE PEN.
I VOTED ‘NO’ SOLELY ON THE PEN DESIGN…
COMMITTEE MEMBER KENNETH D. BURMAN, MD
HAVING THE WORDING OF THE PROPOSED PEN IN THE VOTE WAS A STRUGGLE FOR ME. THE PEN NEEDS TO BE REDESIGNED.
COMMITTEE MEMBER ELLEN W. SEELY, MD
According to Sanofi SA (ADR) (NYSE:SNY), the application is now resubmitted and the FDA has deemed whatever is included in the resubmission as a major alteration. The fact that it is a major alteration automatically initiates a three-month delay. We are guessing that the alteration is the pen design, but as yet, cannot confirm this.
If it isn’t, Sanofi could be in real trouble come decision day. The FDA has two products that are almost identical in efficacy, tolerability and indication, and it may want to approve both to ensure a competitive landscape and patient choice. However, if one is inferior (and right now, Sanofi’s candidate is) then the agency might refuse to take the risk and decline IGlarLixi outright.
Note: This article is written by Mark Collins and was originally published at Market Exclusive.