The first is through the use of a surrogate endpoint. The way this would work is that the trial would use healthy volunteers, and the endpoint would be something that is indicative of efficacy – most likely an immune response.
The second is via what’s called the Animal Rule. This one is pretty much exactly what its name suggests. A company would conduct trials in animals, and then present data to the authorities based on these trials. If the data shows efficacy in the animals in question (most likely primates in this instance) and the agency thinks that the data suggests that this benefit would transmit to humans that undergo the same treatment, it will approve the vaccine conditional on strict post-marketing studies.
Data presented as part of a government-sponsored workshop in the US suggested that immune response was similar in both healthy humans and non human primates (NHP). This points to the potential for a combination of the two above methods being used to garner an approval, and is the most probable path going forward.
The Drugs
There are currently three frontrunners in development of a vaccine, two of which will be covered here. These are:
1. rVSV-ZEBOV
2. ChAd3-EBO-Z
3. Ad26.ZEBOV
As the names of each suggest, they all target the Zaire member subtype, ZEBOV. To date, all have been put through phase I trials, and all have demonstrated some level of efficacy, as measured by immunogenicity, basically the proliferation of immune cells in response to the vaccine, which doesn’t prove that the immune response will actually prevent infection however. The first two candidates on the list, rVSV-ZEBOV and ChAd3-EBO-Z, have published trial data available. The third, Ad26.ZEBOV, does not. As such, for the purposes of this report, we will focus on the two with trial data available going forward.
Phase I Clinical Trial Data
This section of the report will focus on the two vaccines for which there is available phase I data – rVSV-ZEBOV and ChAd3-EBO-Z.
rVSV-ZEBOV
rVSV-ZEBOV was initially developed as part of a program by the Public Health Agency of Canada (PHOC), and then licensed to NewLink Genetics Corp (NASDAQ:NLNK). Subsequent to this licensing, NewLink entered into a collaboration deal with Merck & Co., Inc. (NYSE:MRK), which saw the latter granted exclusive rights to the rVSV-ZEBOV vaccine candidate as well as any follow-on products.
The letters in the name stand for Ebola Virus Recombinant Vesicular Stomatitis Virus-Vectored vaccine, quite a mouthful. It is a vaccine based on a genetically modified vesicular stomatitis virus (VSV) that expresses the envelope glycoprotein (GP) from the ZEBOV strain. So basically they take a different virus, cover it with glycoproteins from Ebola, and it masquerades around as a sort of fake Ebola for the immune system to train on.
Data from a phase I study showed median GP ELISA antibody responses of 1:2,000 at day 28 at a vaccine dose of 106 pfu. ELISA here refers to an enzyme-linked immunosorbent assay. Essentially, these assays measure how much of an antigen is present in a patient’s system post administration. The 1:2,000 number is a pretty robust response, a good sign but not necessarily effective against actual Ebola. The important thing is what’s called seroconversion. Seroconversion is the point at which the body develops antibodies to a level that they are detectable in the blood. Data from two phase I trials conducted at WRAIR and NIH showed a 100% seroconversion rate in 40 patients that received the vaccine.
The chart below taken from the FDA briefing document details the data from four different studies of the rVSV-ZEBOV vaccine across the US, Europe and Africa.
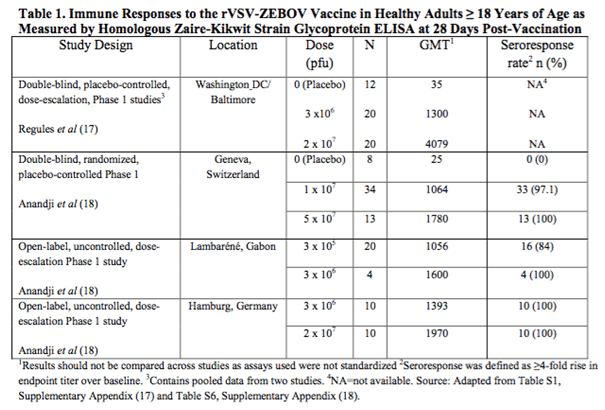
The data is pretty convoluted right now but the interpretation is as follows. As the dose increases, GMT increases. GMT here is geometric mean titer, for which a higher number correlates to a higher response rate. An increased response to an increased dosing is generally a good sign in biotech, primarily because it is a strong indication of efficacy.
Follow Merck & Co. Inc. (NYSE:MRK)
Follow Merck & Co. Inc. (NYSE:MRK)
Receive real-time insider trading and news alerts





